Authors Guideline
Article Types
The International Journal of Pharmaceuticals, Nutraceuticals and Cosmetic Science (IJPNaCS) accepts original research articles, full length review articles, mini reviews, short communications, case reports, and letters to the editor. Manuscripts must be written in a clear and concise manner and should include structured headings (refer to the preparation of manuscript guidelines for details).
General format and length of the types of articles accepted for submission
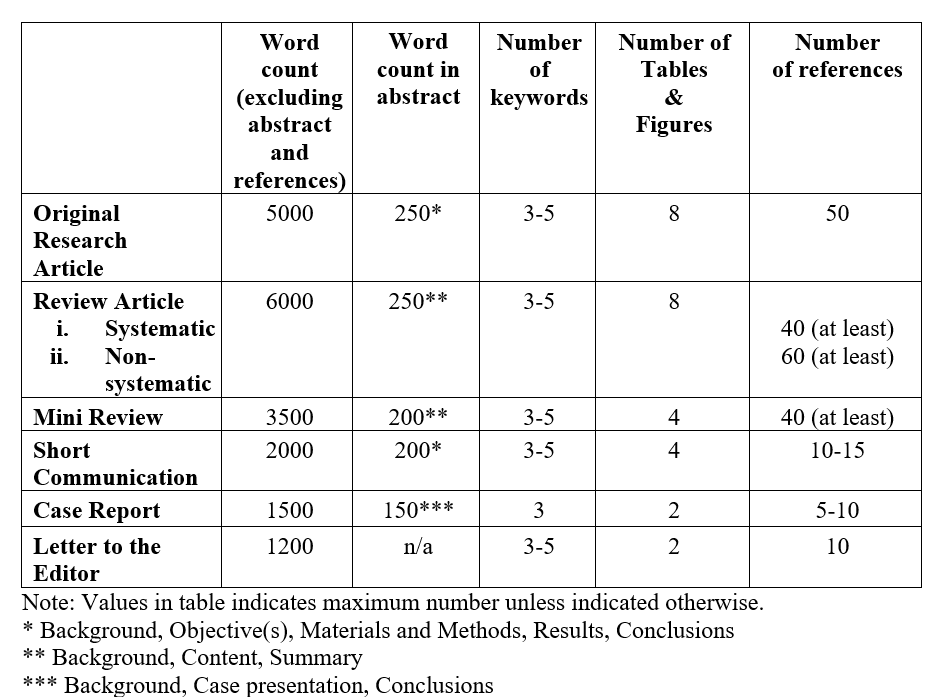
Original research articles
Articles describing substantial original research falls within the scope of the journal. Structured headings are required (refer preparation of manuscript for details).
Review articles should be prepared according to one of the following styles:
- Systematic reviews should contain original articles' parts. The protocol of these studies should adhere to PRISMA (for systematic reviews of RCTs) or MOOSE (for Observational studies) guidelines. Abstract should be structured.
- Nonsystematic reviews (Narrative reviews) should be written by experts contained the critical assessment of the current knowledge of the field. Different parts of these articles include abstract, introduction, discussion and conclusion. Abstract should be structured.
Short communication/Brief reports can be in form of research article, systematic review or ongoing research which reports its interesting findings. The parts in this type of articles are like those of original one but they are smaller and prepared in maximum 2000 words.
Case Reports: Should include abstract, keywords, case presentation, discussion, acknowledgment, references, and figures. Necessary documentations of the case(s) like pathology reports, laboratory test reports, and images should be included in the submission package.
A letter to the editor should be about criticism of previous articles, criticism or review over books, analysis of a related topic, expansion and explanation about an idea or a complicated problem. These articles need no structure.
Preparation of manuscripts
Formatting
- All manuscripts must be in English.
- Manuscripts should be single spaced; the text should be in Times New Roman 12-point font.
- Title page
- The TITLE in BOLD, ALIGN LEFT (14-point font) with a 25 mm top margin, followed by the names of Author(s) separated from the title by a single line space, align left and indicating by an asterisk* the author to whom correspondence should be addressed. To assist information retrieval, titles should be concise and informative.
- The full correspondence addresses (align left), should be separated by a single line space from the author names.
- Avoid abbreviations, symbols or formulae in your title.
- Abstract
- An informative Abstract (heading align left).
- The abstract should briefly state the purpose of the study, the principles results and major conclusions.
- Keywords: (heading align left) continuing the same line as the heading. Provide a maximum of 6 keywords.
- The text should normally be divided into conventional Introduction, Materials and Methods, Results, Discussion and References. These headings should be BOLD, ALIGN LEFT, NUMBERED, and separated from the text by one line above and below. Subsections should be numbered 1.1 (then 1.1.1, 1.1.2,……), 1.2, etc.
- Introduction (summarize the purpose and the rationale for the study);
- Materials and Methods (study design and exact method or observation or experiment, definitions such as for diagnostic criteria, the population or patient samples, and laboratory and statistical methods);
- Results (presented in the form of text, tables and illustrations)
- Discussion (present findings and the variations or similarities with other work done in the field by other workers)
- Conclusion (final result that the author(s) has (have) reached)
- Acknowledgements (All contributors who do not meet the criteria for authorship should be covered in the acknowledgement section. Financial and material support should also be acknowledged)
- Conflict of interest and
- References.
- Acknowledgements: (heading align left) if any, should appear after the text continuing the same line as the heading.
- The text should be fully justified and continuous with no spaces between paragraphs, the first line of which should be indented approximately four spaces. Subheadings should be in lower case regular italic with Initial capital.
Article Manuscript Submission Template For submitting article, please download the template at https://docs.google.com/document/d/1xZgX6ENwsIX6y5HO39xbiMEDJss9v-tV/edit
Statistical analysis
Experimental design, subject selection and randomization procedures should be described, and analytical precision quoted when appropriate. Appropriate power calculations for the sample size used should be given. In case–control studies clearly define how cases and controls were selected and what matching has taken place. Authors should detail how they have addressed missing data and loss to follow up. Analytical methods used to account for sampling strategy should be described. Rounded figures are easier to compare, and extra decimal places should be avoided. Descriptive statistics require an additional digit to those used for the raw data. Percentages should not be expressed to more than one decimal place and should not be used at all for small samples.
Normally distributed data should be described using a mean, SD and/or %CV and expressed as ‘mean (SD)’ or ‘mean ± SD’. When data are not normally distributed, then medians and interquartile ranges should be used in place of mean and SD. Skewed data can often be normalized by logarithmic transformation or a power transformation. The statistical analysis and calculation of summary statistics should be carried out on the transformed data and the summary statistics transformed back to the original scale for presentation. If a logarithmic scale is used, then graphs should display non-transformed data on a logarithmic scale. The conventional use of statistical significance is P≤0.05. If a different significance level needs to be used, then the reasons why must be clearly stated in the statistical method section.
Abbreviations and units
Abbreviations: Define abbreviations and acronyms the first time they are used in the text, even after they have been defined in the abstract. Symbols and abbreviations should be those currently in use. Authors should not create new abbreviations and acronyms. Do not use abbreviations in the title or headings unless they are unavoidable.
Units: All measurements should be expressed in SI units.
Artwork, figures and other graphics
Illustrations, pictures and graphs, should be supplied with the highest quality and in an electronic format that helps us publish your article in the best way possible. Please follow the guidelines below to enable us to prepare your artwork for the printed issue as well as the online version. Rasterized based files (i.e. with .tiff or .jpeg extension) require a resolution of at least 300 dpi (dots per inch). Line art should be supplied with a minimum resolution of 800 dpi.
Tables
In limited numbers, they should be submitted with the captions placed above and on separate pages. Each table should be numbered consecutively with Roman numerals and typed single-spaced, including all headings. Verify tabular statistics to make sure they tally and match the data cited in the text. Do not submit tables as photographs. Please add a placeholder note in the running text (i.e. “[insert Table 1.]"). Insert tables after they are cited in the text.
Figures
Should be in limited numbers, with high quality artwork and mounted on separate pages. The captions should be placed below the figures. The same data should not be presented in tables, figures and text, simultaneously. Please add a placeholder note in the running text (i.e. “[insert Figure 1.]"). Insert figures after they are cited in the text. Use the abbreviation “Fig.1”, even at the beginning of a sentence.
Illustrations
All illustrations must be numbered as cited in the text in consecutive numeric order. Written permission must accompany any photograph in which the subject can be identified or any illustration that has been previously published.
Supplementary material
This journal may host additional materials online e.g. movie clips, questionnaires.
Conflict of interest and other declarations
All authors are required to declare any conflicts of interest when submitting papers for publication. Declarations of funding sources, ethical approval and acknowledgement should be included at the end of the manuscript (before references list).
- Declaration of competing interests: GKSS is an employee of XYZ Coorperation. GS provides consultative advice to ABC corporation.
- Funding: This research was funded by (please provide grant name and number)
- Ethical approval: The animal ethics committee of UiTM (UiTM CARE) approved this study (please provide number)
- Acknowledgements
Reference style
All manuscripts should be accompanied by relevant references. The Reference should provide the following information as stated in the presented models as follows:
References should be numbered sequentially as they appear in the text according to the Vancouver style. When citing authors in the text, acknowledge only the first author where there are three or more authors, e.g. Williams et al (1) stated that.... Where there are two authors cite both, e.g. Jones and Smith (2) reported that.... Citations in the reference list are to be arranged by number in the following format including punctuation.
Reference list should be single spaced with one line spaced between each entry.
Journals: Author(s). Title of article. Title of journal. Year; Volume number: Page numbers. (Abbreviations for journals used in the reference list should conform to Index Medicus.)
Books: Author(s). Title: sub-title. Edition. Place of publication: Publisher; Year:pages numbers.
Chapter in a book: Author(s) of chapter. Title: sub-title of chapter. In: Author(s) (or editors) of the book. Title: sub-title of book. Place of publication: publisher; Year; page numbers.
E-books: Author (s). Title of e-book: subtitle [format]. Place: Publisher; Date of original publication [cited year abbreviated month day]. Available from: Source or URL.
Internet documents: Author (s). Document title. Webpage name [format]. Source/production information; Date of internet publication [cited year month day]. Available from: URL.
Newspaper articles: Article Author (s). Title of article. Title of Newspaper: Section. Year Abbreviated Month Date: inclusive page numbers.
Conference paper from the internet: Author (s) of paper. Title of paper. In: Proceedings of the Title of Conference: subtitle of Conference; Year Month Date; Location. Place of publication: Publisher; Year. Available from: URL or Database Name.
Conference proceedings: Editor A, Editor B, editors. Title of conference: subtitle of conference; Year Month Date; Location. Place of publication: Name of Publisher; Year.
Theses (full text database): Author. Title of thesis: subtitle. Thesis type [format]. Location of University: University; Year. Available from: Database Name.
Ethical conduct of research involving human and animals
The research that involves human subjects must adhere to the principles of the ethical standards of the responsible committee on human experimentation (institutional and national) and World Medical Association Declaration of Helsinki. All research participants should be informed about the aims of the study and any possible side effects of the drugs and intervention.
When reporting experiments on animals, authors should indicate whether the institutional and national guide for the care and use of laboratory animals was followed.
Written informed consent under protocols approved by an institutional or local review board or approved animal protocols are essential if the research involves human or animal subjects, respectively. This information should be stated in the manuscript and the protocol number or exempt status of approved protocols should be stated in the manuscript at the time of submission for review.
Ethical considerations must be clearly addressed in the Declaration or the Methods section, and the name of the appropriate institutional review board that approved the project should be mentioned. The International Journal of Pharmaceuticals, Nutraceuticals and Cosmetic Science reserves the right to request the related documents.
Manuscript Revision
The cover letter that accompanies your revised manuscript must include a point-by-point response to each comment by the reviewers and editor(s). Typically, this is addressed by listing each comment verbatim, and then directly following each comment with an explanation of how you addressed it, with reference to specific line or figure numbers in the revised manuscript. Using a different colour or font for the review comment vs. your response or inserting the review comments and your responses into a two-column table is strongly encouraged. Should you disagree with the constructive criticism raised, it is imperative that you provide a clear rationale for why you disagree in the point-by-point response.
Revised manuscripts and the point-by-point cover letters are submitted to ijpnacs@uitm.edu.my


